Installation
The easiest way to get metalite.ae is to install from CRAN:
install.packages("metalite.ae")Alternatively, to use a new feature or get a bug fix, you can install the development version of metalite.ae from GitHub:
# install.packages("remotes")
remotes::install_github("Merck/metalite.ae")Overview
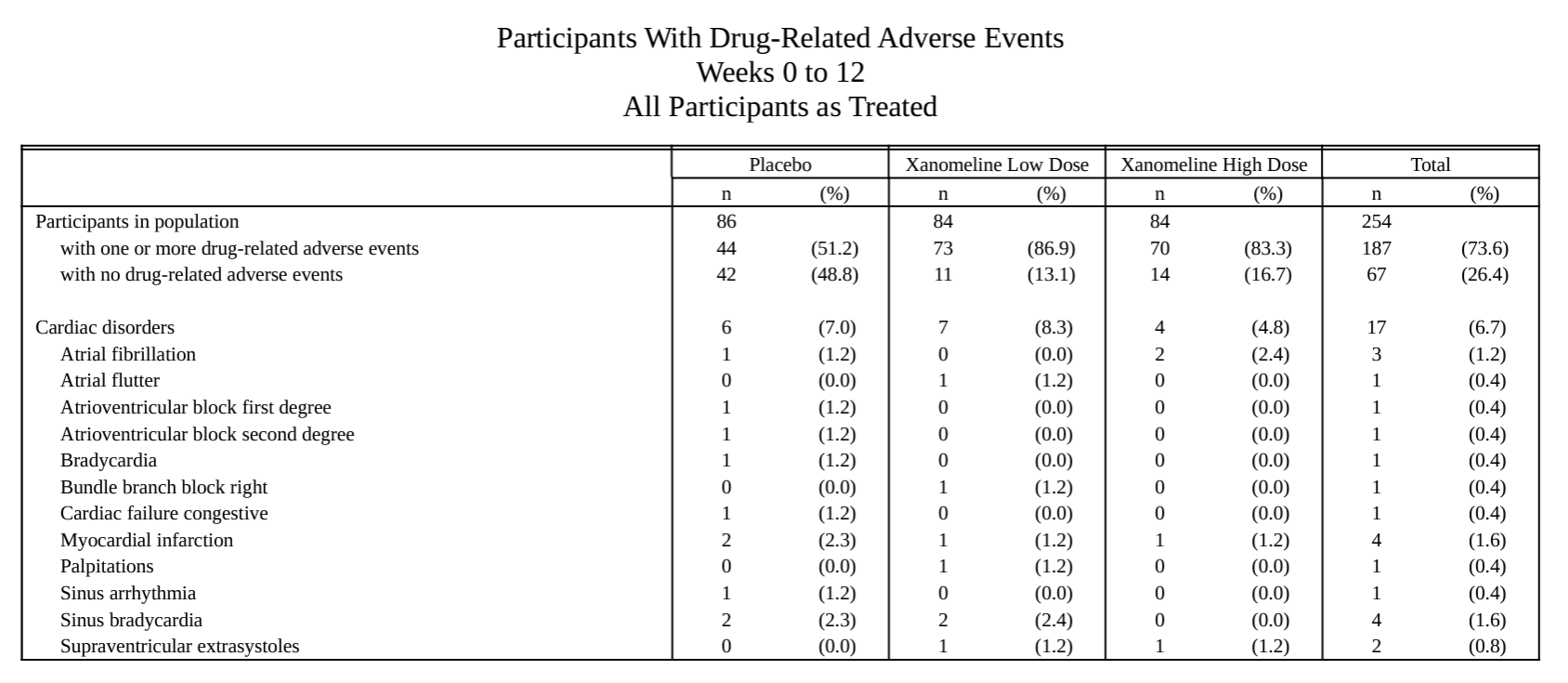
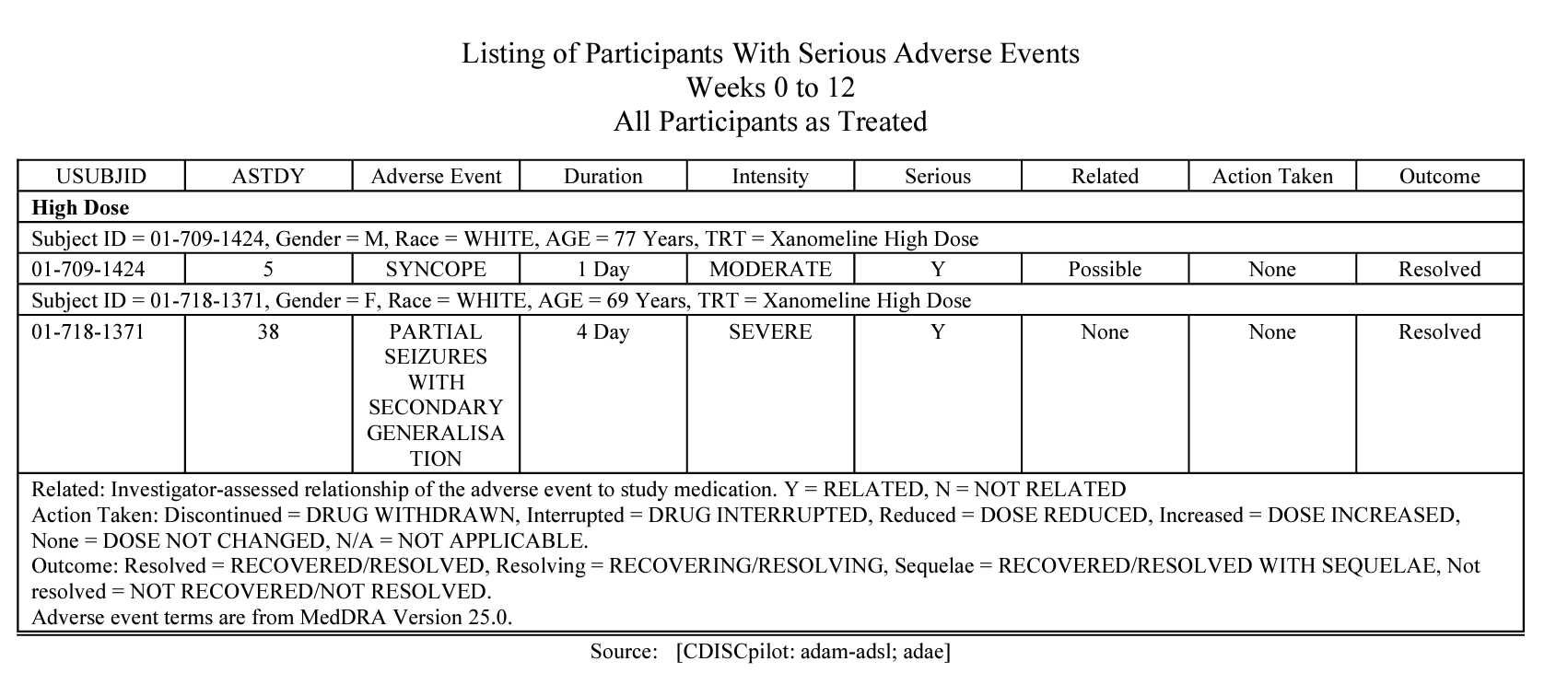
metalite.ae is an R package designed for the analysis of adverse events (AE) in clinical trials. It operates on ADaM datasets and adheres to the metalite structure. The R package streamlines the process of generating production-ready tables, listings, and figures as outlined in the AE summary chapter and the specific AE chapter of the R for Clinical Study Reports and Submission book. The package encompasses the following components:
Highlighted features
- Avoid duplicated input by using metadata structure.
- For example, define analysis population once to use in all adverse events analysis.
- Consistent input and output in standard functions.
- Streamlines mock table generation.
Example
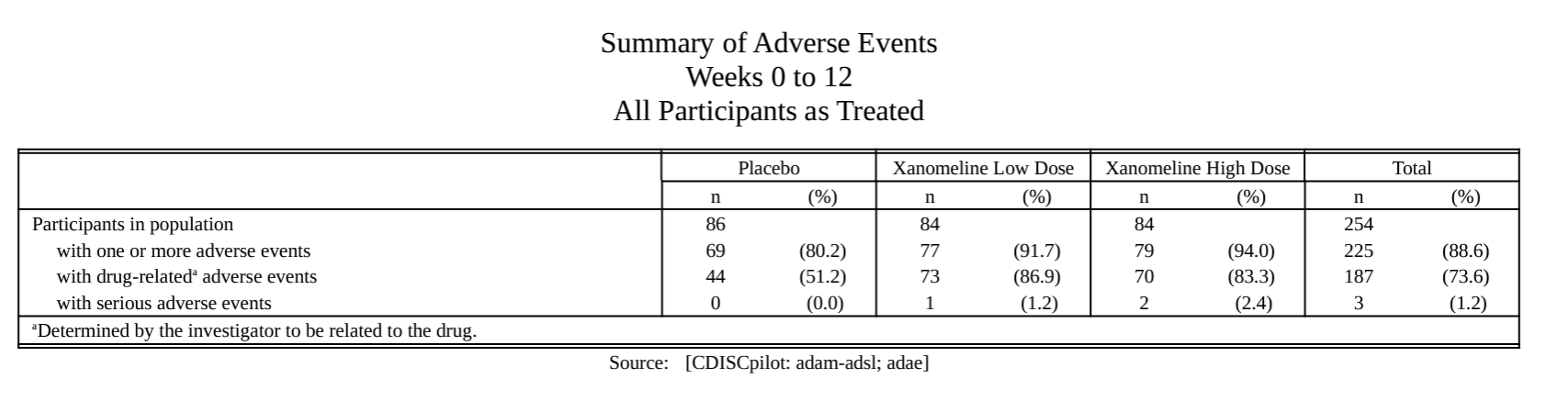
meta_ae_example() |> # Example AE data created using metalite
prepare_ae_summary(
population = "apat", # Select population by keywords
observation = "wk12", # Select observation by keywords
parameter = "any;rel;ser" # Select AE terms by keywords
) |>
format_ae_summary() |>
tlf_ae_summary(
source = "Source: [CDISCpilot: adam-adsl; adae]", # Define data source
path_outtable = "ae0summary.rtf" # Define output
)