Overview
metalite.ae is an R package designed for the analysis of adverse events (AE) in clinical trials. It operates on ADaM datasets and adheres to the metalite structure. The package encompasses the following components:
AE summary.
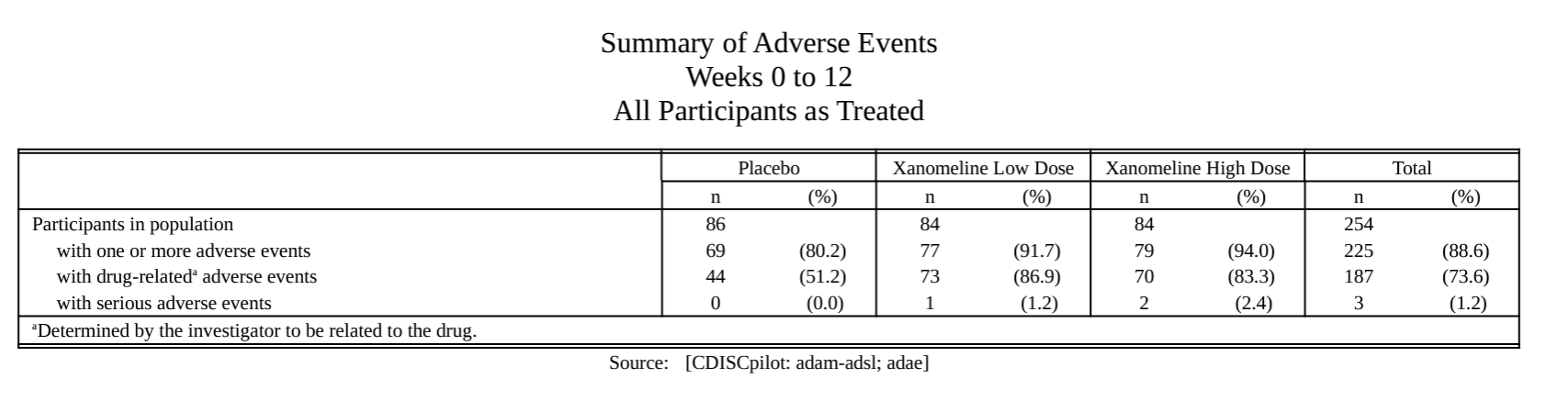
Specific AE analysis.
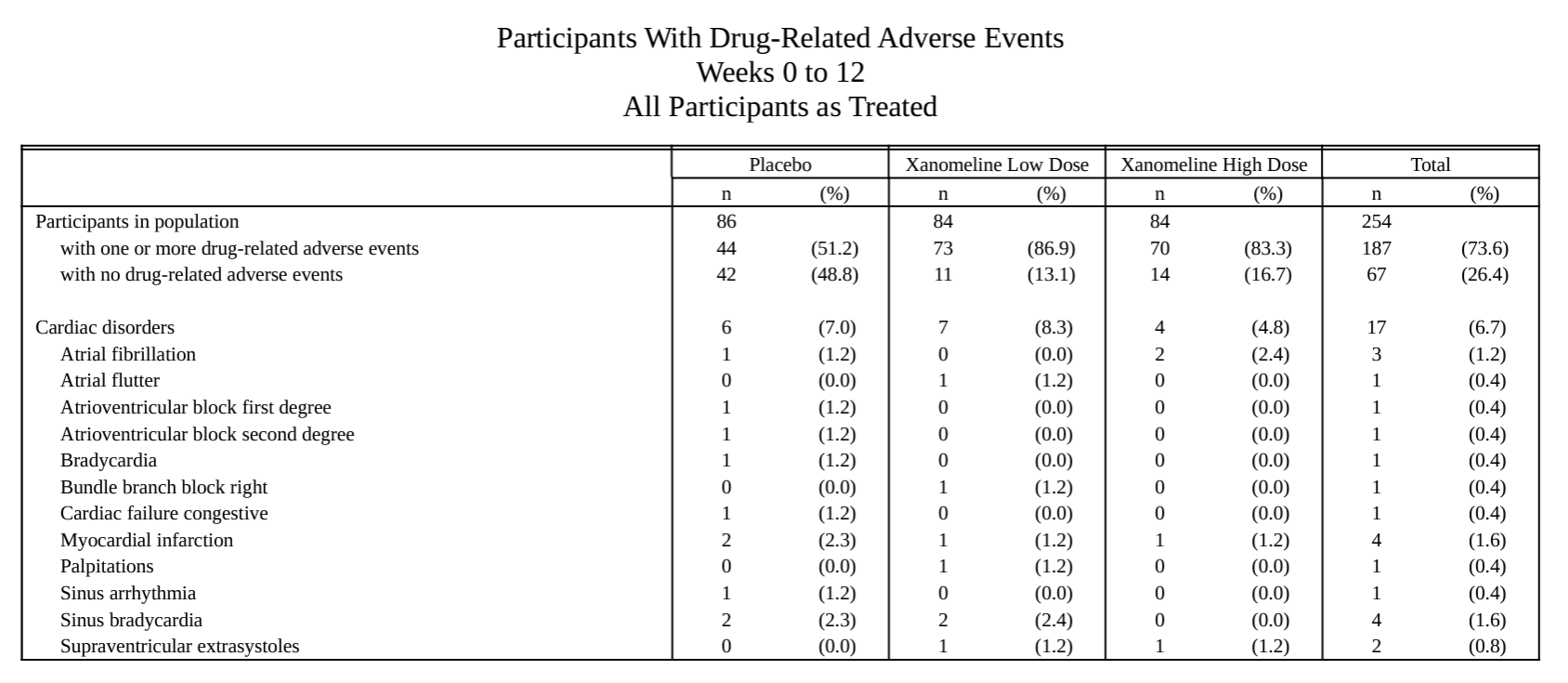
AE listing.
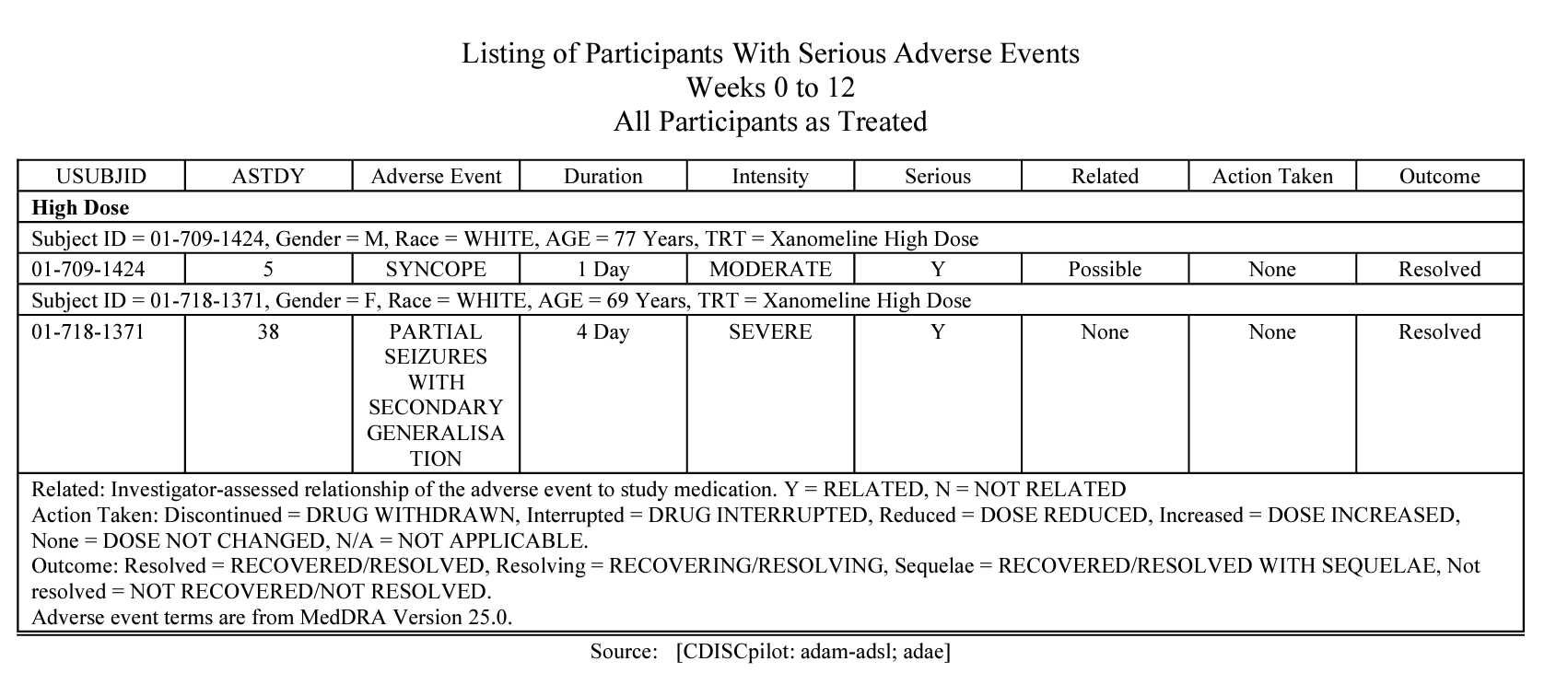
The R package streamlines the process of generating production-ready tables, listings, and figures as outlined in the AE summary chapter and the specific AE chapter of the R for Clinical Study Reports and Submission book. It ensures complete traceability throughout the development lifecycle, leveraging the metalite data structure.
This R package offers a comprehensive software development lifecycle (SDLC) solution, encompassing activities such as definition, development, validation, and finalization of the analysis.
Highlighted features
- Avoid duplicated input by using metadata structure.
- For example, define analysis population once to use in all adverse events analysis.
- Consistent input and output in standard functions.
- Streamlines mock table generation.
Workflow
The overall workflow includes the following steps:
- Define metadata information using metalite R package.
- Prepare outdata using
prepare_*()functions. - Extend outdata using
extend_*()functions (optional). - Format outdata using
format_*()functions. - Create TLFs using
tlf_*()functions.
For instance, we can illustrate the creation of a straightforward AE summary table as shown below.
meta_ae_example() |> # Example AE data created using metalite
prepare_ae_summary(
population = "apat", # Select population by keywords
observation = "wk12", # Select observation by keywords
parameter = "any;rel;ser" # Select AE terms by keywords
) |>
format_ae_summary() |>
tlf_ae_summary(
source = "Source: [CDISCpilot: adam-adsl; adae]", # Define data source
analysis = "ae_specific", # Provide analysis type defined in meta$analysis
path_outtable = "ae0summary.rtf" # Define output
)Additional examples and tutorials can be found on the package website, offering further guidance and illustrations.
Input
To implement the workflow in metalite.ae, it is necessary to
establish a metadata structure using the metalite R package. For
detailed instructions, please consult the metalite
tutorial and refer to the source code of the function meta_ae_example().
